Left-click: rotate, Mouse-wheel/middle-click: zoom, Right-click: pan, Escape: close
sadly this exists
Key Messages + 1 Section
- Strict water quality controls are in place to protect human health and aquatic ecosystems from chemical and biological pollutants.
- In general, control of pollutants at their source is more effective than remediation because of their persistence in the environment and concentration through the food chain.
- Elevated levels of:
- salinity
- nutrients
- metals
- pathogens
- organic contaminants (e.g. pesticides)
- result in low water quality in Australia
- Pollutants result from agriculture, industry and urban areas (wide range of souces) (most prevalently ag + urb, due to catchment land use)
- Sediment layers at the bottom of waterways are a major sink for nutrients and contaminants, which can be released into waters and become toxic under certain conditions.
- New contaminants, for example pharmaceuticals, are continually emerging and much monitoring and research is focused on detecting their presence and toxicity in aquatic environments.
- Either water treatment or the protection of sources (e.g. water supply catchments for Perth)
- Pollutants may enter the food chain
- -> fisheries depend on water quality
- -> agricultural productivity may be affected
- -> some aquatic ecosystems may be damaged due to low water quality
Main nutrients in water:
-
phosphorus
-
nitrogen
-
cations
-
trace metals
-
biological constituents (living things)
-
Physical properties of water (temp, light penetration(?)) affect survivability of aquatic organisms
e.g. Dams release water into rivers from the top, as the lower-most water is too cold/deprived of oxygen.
- Ecosystems adapt to natural water quality
- Changes in water quality can greatly affect survivability/life
Pollution:
- Can result from changes in the naturally occurring concentration of some components in water
- e.g. nutrient levels too high, algae grows toxically
- Most commonly, oxygen levels go too low
- Also result from man-made constituents that do not naturally occur in water
- e.g. pharmaceutical products leak into river
**Steps to manage pollution in river basin/groundwater system:
- Define use/value of water + risk to said use/value due to pollution
- Sources of pollution should be identified (+pathways in which the pollution may reach the water)
- For large water supply catchments, there may be multiple sources, and pollutants (chem/bio only) can transform as they are transported
- e.g. herbicides can degrade to be harmless, thus they only act as pollutants close to the water source
- Targets for water quality are set, and actions to achieve them
- Water quality should be monitored so pollution risks can be identified, and to evaluate effectiveness of present pollution management strategies.
- Water from point source pollution (when pollutants are discharged e.g. pipe, factory) has improved recently due to better regulations.
- This is an example of effective pollution management strategies.
Examples of point source pollution
-
Industrial plants
-
Hospitals
-
Sewage treatment plants
-
Mine sites
-
Widespread pollution of water (diffuse pollution) from usage of land where water is collected (catchment land use) is hard to combat
- This is currently the biggest water pollution problem today
-
Examples of catchment land use are for agricultural and urban land use.
Point Source Vs Diffuse Pollution:
Point source: Pollution that is discharged from a single source (e.g. a discharge pipe).
Diffuse: The release of pollution from multiple sources that individually may not affect water quality
Important!! /\
Examples of pollution from catchment land use
- Salt
- Nitrogen
- Phosphorus
- Suspended sediment (?)
- Note that some are natural constituents of water, therefore the concentration required to prevent ecological damage are variable (+ hard to determine)
Salinity
- Affects a lot of agriculture.
- Salinity is ultimately caused from rainfall (ocean spray in rainfall)
How to reduce
- Revegetation of water catchments
- Using pastures (grazing land for animals)
- Salt interception schemes pump salt to be evaporated/stored
- Quality irrigation practices
- The effect of salinity is more pronounced during wet years.
Insert Pearson here: Obtaining drinking water from seawater
- Use distillation or reverse osmosis
- hey christian instead of reading this in github why dont you get a zip file and download it so you can properly search for distillation notes because im not gonna write them here for you
- Osmosis occurs when water moves from a region of low salt concentration to high salt concentration.
- If there exists a semipermeable membrane that allows water but not the salt ions, then water will leave from the fresh water to salt water.
- However when pressure is applied to the salt water, water is pushed through the membrane, leaving just the salt water.
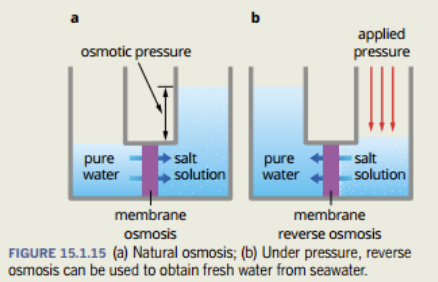
- The problem with reverse osmosis is finding membranes that are durable and do not break under the high amounts of pressure.
Algal Blooms
- Algae provide food for aquatic wildlife
- Examples include phytoplankton, cyanobacteria, diatoms and seaweed.
- Eutrophication: Water sources become enriched in nutrients such as nitrogen and phosphorus
- This leads to algae overgrowth(known as algal blooms), and the production of blue-green algae, which is toxic to animals and people that come in contact.
- When algal blooms decompose, dissolved oxygen in water is consumed, resulting in the death of fish.
- **Importantly, light, turbidity(opacity of water) and water stratification (when water separates into distinct levels) are the most important triggers for algal blooms. **
- In Australia, water stratification occurs readily. This allows for more algal blooms.
- Turbidity benefits toxic algal blooms, as they float up and out-compete other algae struggling for light.
- Managing local conditions provides short-term help, rather than reducing the run-off of sediment, nitrogen and phosphorus (i.e. reducing eutrophication)
- Ways to combat algal blooms:
- Water can be mechanically stirred, this increases oxygen and reduces stratification
Acid sulfate soils explained

Microscopic image of pyrite in soil
Southern Cross University
Common in many parts of the world, acid sulfate soils are saturated with water, almost oxygen-free and contain microscopic crystals of iron sulfide minerals (commonly pyrite).
Acid sulfate soils are safe and harmless when not disturbed. If acid sulfate soils are dug up or drained they come into contact with oxygen. The pyrite in the soil reacts with the oxygen and oxidises.
This process turns pyrite into sulfuric acid, which can cause damage to the environment and to buildings, roads and other structures.
The acid also attacks soil minerals, releasing metals like aluminium and iron. Rainfall can then wash the acid and metals from the disturbed soil into the surrounding environment.
Pearson Notes
15.1 Essential water
-
Sources of chemical contamination:
- Run-off from farms and cities
- Run-off from industrial and mining wastes
- Lead used in solder in copper water pipes
-
Types of chemical contaminants:
- Heavy metals
- Pollutants from fertilisers
- Organic pollutants
-
Heavy metal contamination
- The main heavy metals are mercury and lead
- Other heavy metals:
- Copper
- Lead
- Cadmium
- Nickel
- Zinc
- Arsenic
- Mercury
- Common effects: Cancer, organ/nervous system damage, death
- Mining is a main example of heavy metal contamination
- Another one is water pipes
- How to remove: precipitation reaction
- Heavy metal cation is identified
- A soluble compound containing an insoluble compound with the cation of the heavy metal is selected
- A solution of the compound is added to the water
- A precipitation with the heavy metal is produced
- Precipitate is filtered off
-
Water monitoring:
- Protocols for water sampling:
- The container can be rinsed with the sample before the final sample is taken
- The container should be cleaned before taking another sample
- The water used for cleaning the container can be tested to ensure there is no contamination between samples.
- Note that if pathogens are being tested for, the container should be sterile
| Condition | Sampling method |
| ------------------------------------------------ | ---------------------------------------------------------------------------------------------------------------------------------------------------------------- |
| sample taken from a well-mixed body of water | sampling near the surface is sufficient to obtain a representative sample. A sample should be taken about 10cm below the surface and away from the water's edge. |
| sample taken from water contaminated by sediment | a sample should be drawn into the sample container by suction to avoid including the sediment |
| sample taken from a river | a sample should be taken upstream from where the person taking the samples stands |
-
How to determine depth for sampling:
- Measure temperature at every metre.
- If temperature is consistent, assume water is mixed, thus take sample halfway down.
- If there is temp variation, take sample in middle of each temperature region.
- If deeper than 2m, use Van Dorn sampler.
- Useful for distinct layers
- Use many locations
-
Treatment of drinking water
- Steps for purification:
- Flocculation
- Settling of the floc
- Filtration
- Chlorination
- Fluoridation (sometimes)
- Flocculation: process where small suspended particles in the water are made to join together to form larger, heavier particles.
- Heavy particles sink
- $Ca(OH)_{2}$ and $Al_2(SO_4)_3$ (Alum) is added to water.
- The dissociation of these two substances produces Aluminium hydroxide ($Al(OH)_3$).
- $Al(OH)_3$ is a gelatinous precipitate and is known as floc.
- This floc traps finer particles, and can remove colour/microorganisms from water, as they are absorbed by $Al(OH)_3$.
- Floc particles coagulate to create heavier particles.
- Sedimentation: Using gravity, floc settles into a sludge that accumulates at the bottom of the container (settling tank) and is then removed, while the water proceeds to filtration.
- Filtration: The water is passed through a layer of sand on top of a layer of gravel. This removes any remaining suspended matter
- Chlorination: Gaseous chlorine is added to kill any biological contaminants.
- After this process, water is now fit for human consumption. However, water may also undergo fluoridation.
- Fluoridation: Fluoride ($F^{-}$) is added to drinking water before it is released from storage.
- This is done by adding compounds such as:
- Sodium hexafluorosilicate ($Na_2SiF_6$)
- Fluosilicic acid ($H_2SiF_6$)
- Sodium fluoride ($NaF$)
- They break down into Fluoride ions in water.
- Fluoride reacts with tooth enamel to produce fluorapatite, a stronger more resistant compound. In doing so, tooth decay is reduced.
15.2 Properties of water
- hydrogen bondssssss ::::::::::::::::::::::::))))))))))))))))))))))))))))))))))))))))
- Density in the liquid and solid states:
- Water expands as it freezes.
- Water has a lower density as a solid than as a liquid.
- In the liquid state, water molecules move randomly and freely with some hydrogen bonds.
- In the solid state, water forms large hexagonal lattices, which take up more space. Thus water expands, and has a lower density as it freezes.
- High surface tension:
- At the surface of water, molecules are not encased in other water molecules. As a result, hydrogen bonds form between molecules to their side and below them, but not above.
- The sideways forces result in a balance, however due to there being hydrogen bonds below the molecule and not the top, there is an imbalance and molecules at the surface are pulled downwards towards the bulk of the water molecules.
- This downwards force is very fast, and molecules at the surface only spend $1 \times 10^{-9}$ seconds or 1 nanosecond before being pulled into the bulk of the liquid.
- Surface tension is a measure of the resistance of a liquid to increase its surface area.
- As a result of water particles at the surface being pulled towards the bulk of the liquid, water tends not to increase surface area as that would lead to more molecules being pulled towards the bulk, which is energetically unfavourable.
- Thus, water has a high surface tension.
- Heat capacity:
- Heat capacity - a measure of a substance's capacity of absorb and store heat energy.
- When heated with a specific amount of energy, water tends to change temperature slower than other substances, such as ethanediol.
**Specific heat capacity:** the amount of energy (in joules) needed to increase the temperature of a certain amount (usually 1 gram) of a substance by 1 $\degree C$
- Units for specific heat capacity: $J \ g^{-1} \ \degree C^{-1}$
- Specific heat capacity reflects the type of bonding. For covalent substances, it reflects the strength of intermolecular forces between molecules.
- Equation for calculating specific heat capacity: $q = C \times m \times \Delta T$
- q = amount of heat energy
- C = specific heat capacity (of the substance)
- m = mass
- $\Delta T$ = temperature change
- When you heat a substance to change its state of matter, temperature would increase at it approaches the melting point, then it remains constant as the solid melts, then temperature increases as it reaches the boiling point, and once again remains constant as the liquid evaporates.
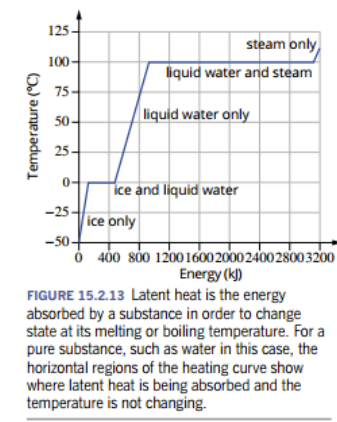
- Latent heat is the energy required to change a fixed amount of substance, from a solid to liquid or liquid to gas. During this period, temperature does not change
- Latent heat of...
- Fusion: heat needed to change 1 mol of substance from solid to liquid at melting point
- Vaporisation heat needed to change 1 mol of substance from liquid to gas at boiling point
15.3 Water as a solvent
- The more polar a compound is, the more likely the compound is to dissolve in water.
References
- Chapter 5 “Water Quality” from Water: Science and Solutions for Australia Prosser, I. (2017). Water (pp. 76 - 89). Collingwood, Vic.: CSIRO Publishing.
- Pearson Chapter 15
- Pranav Borude
- Me (i made stuff up, deal with it)


