Period number is equal the the electron shell that an element's valency electrons occupy. E.g. in period 2, the valence electrons are on the n = 2 shells.
Groups have the same valence electron number, and this will dictate their chemical properties / the ways they bond.
Note: They are similar but not the same. Some are more/less reactive than others.
Most elements will try to reach stability of nearest noble gas.
E.g. Potassium has an electronic configuration of $2,8,8,1$, it will achieve stability by losing electrons to have the electron configuration of its nearest noble gas (Argon).
As you go lower on a group, elements get more reactive.
This is because attraction to nucleus is lower as the number of shells increase (and the distance from the energy levels and nucleus increases).
To become an ion you require ionisation energy.
Ionisation energy is needed to break the attraction between an electron and its nucleus, so the further away an electron is from its nucleus the weaker the attraction force, and thus less ionisation energy is required to ionise.
Thus, elements in higher periods are more reactive.
Therefore, elements in higher periods have lower ionisation energy.
Non-metals typically gain electrons.
Typical behaviour of group 7 (non-metals) is to gain electrons.
Check for diatomic molecules!!
e.g.
$Cl_{2}+ 2\bar e \rightarrow 2Cl^-$
What is charge of nitride
Nitrogen has an electron configuration of $2,5$. Therefore, if it wants to reach stability via its nearest noble gas (Neon), which has an electron configuration of $2,5$, it must gain 3 electrons, becoming negative.
$N_{2}+ 6 \bar e \rightarrow 2N^{-3}$ - this is the reduction reaction for nitrogen. redox be like
The actual electron description formula (?) is:
$N+ 3 \bar e \rightarrow N^{-3}$
Note here we are simply describing the redistribution of electrons for one nitrogen atom. Realistically, if this were to happen in nature it would involve 2 atoms, as Nitrogen is diatomic.
Because nitrogen has a smaller atom, and when nitrogen gains electrons, the nucleus's attraction to the electron will be stronger than, for example, phosphorus, as the nucleus' attractive force will be stronger as distance decreases.
Principle quantum number is the term for n.
A new energy level of shell is started at the beginning of each new Period.
Period number is the principle quantum number of the shell being filled.
Group number is the number of electrons in the outer shell. (somewhat, not including the transition metals)
To determine whether something is polar or non-polar, you have to take into account the following:
Looking at symmetry of charges, not the symmetry of molecules.
Dipole is cancelled.
Dipole refers to the slightly negative/positive areas of a molecule
Periodic Trends:
Ionisation Energy:
e.g. $X_{g} \rightarrow X^+_{g}+\bar e^-$
The first ionisation energy of an element is the energy required to remove one mole of electrons from one mole of gaseous atoms.
The first ionisation energy is therefore a measure of the strength of the attraction between the outermost electron and the nucleus.
Effective nuclear charge = Nuclear charge - shielding.
Going across a period, nuclear charge increases.
When atomic radius decreases, electrons become closer and closer to the nucleus. Thus, it would be difficult to remove electrons from an atom.
There is a general increase in the first ionisation energies across periods.
Across period 3, the proton number increases but the amount of shielding does not change significantly. The effective nuclear charge increases.
The greater attraction between the nucleus and the outermost electrons mean
Outer electrons are closer to the nucleus, thus they will experience stronger nuclear attraction, and thus will require more energy to remove from an atom.
Always mention more/less energy required!!!!
As you move along the period, e.g. from Sodium to Argon,
Nuclear charge increases.
Atomic radius decreases?!!!
The principal quantum number (energy shell) stays the same for elements in a period.
Increasing the number of electrons in the inner shells will shield outermost electrons from occupying the outermost principal quantum number .
As nuclear charge increases, electrons in n=1 become closer. This applies for all other shells.
e.g. Na has an atomic charge of 11. In comparison, Argon has a charge of 18. Because of this, it will attract the electrons in n=1 better (electrostatic attraction increases)
Electronegativity: Is the ability for an atom to attract a bonding pair of electrons in a covalent bond.
Covalent bond: shared pair of electrons in a chemical bond
Valence electrons of non-metals that are not shared in the chemical bond are called lone pairs. /non bonding
HCl is a polar molecule.
Hydrogen has no shielding, whereas chlorine has 10 electrons of shielding. Thus, hydrogen's nucleus is more attracted to chlorine's electrons than chlorine is to its. Therefore, chlorine pulls hydrogen towards its nucleus closer than hydrogen pulls chlorine.
We would write chlorine as $\delta -$ , as since it pulls hydrogen closer the electron shared is closer to chlorine, and we write hydrogen as $\delta +$, as its partially lost its electron.
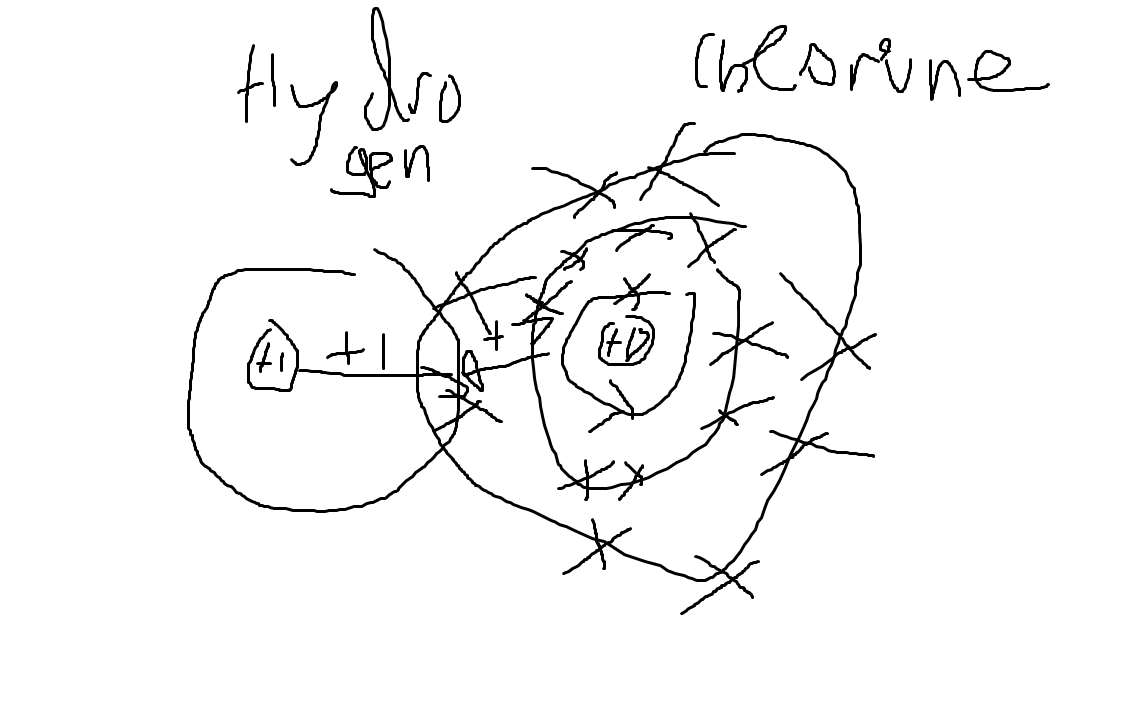
Here, hydrogen has a weaker positive nuclear attractive force than chlorine (10 of its charge is shielded by it), so it does not attract the bonding pair as much as chlorine
==Patterns in the Periodic table:
==Across the period, atomic radius will decrease. (increasing electrons will increase attraction between them and nucleus, decreasing radius)
==Going down groups, atomic radius will increase. (shells are being added).
==Larger atoms mean that valence electrons can be lost more easily, as they are further away from the nucleus and thus experience less weak nuclear attraction force.
==Valency:
==Valency is the combining power of an atom and is equal to the number of hydrogen atoms it could combine with or displace from a compound. (Hydrogen has a valency of 1)
==Valency and number of valence electrons are not the same thing
==e.g. Nitrogen:
==Principle quantum shells - refers to the shells of an atom. e.g. Sodium's principle quantum shells represents 2,8,1.
==Nuclear attraction is derived from the nuclear charge of an element minus the number of inner electrons shielding the outer electrons.
==Electronegative - higher density of electrons
==Going across a period will increase nuclear charge!