Left-click: rotate, Mouse-wheel/middle-click: zoom, Right-click: pan, Escape: close
Key Definitons
- Ground state: the lowest energy state of an atom where all electrons are as close to the nucleus as possible. All other energy states are called excited states.
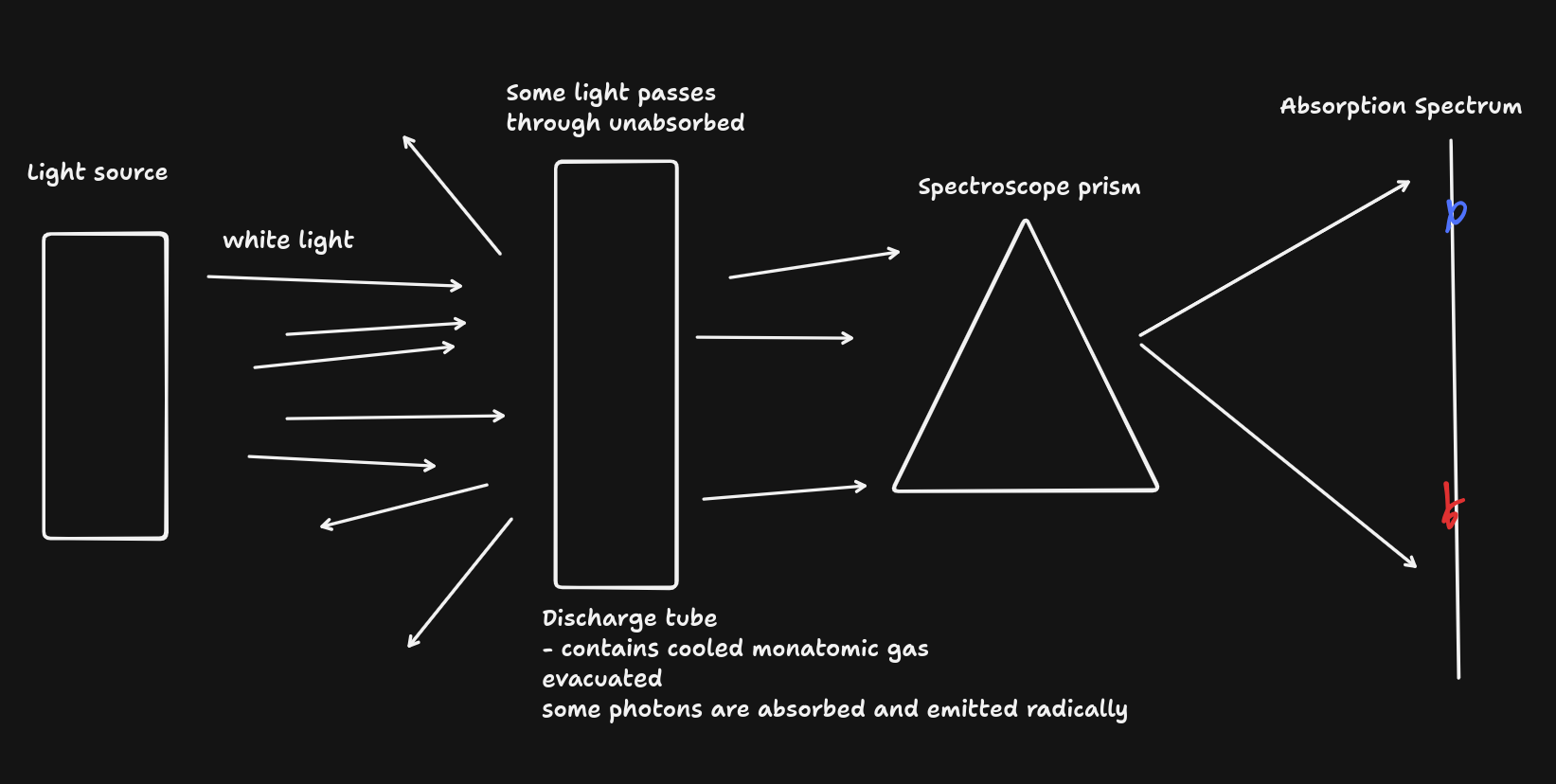
- Line emission spectrum
- The specific range of EMR with discrete frequencies and energies that appears as single lines in an otherwise blank spectrum.
- Produced when a monatomic cooled gas
- is excited by heating or electrons in a discharge tube causing electrons to transition to a higher energy level when they absorb energy that is exactly equal to the energy difference between their original and new energy level,
- producing photons with discrete frequencies
- Band emission spectra
- An emission spectrum composed of broad bands of EMR with very similar energies and frequencies.
- Only produced when complex molecules undergo excitation and de-excitation.
- Molecules have a large number of energy levels (unique of each element, multiple elements in a complex molecule) which are close to each other.
- When excitation and de-excitation occurs multiple photons are produced with very close frequencies
- Thus they appear close together in an emission spectrum resulting in a cluster of spectra lines (bands)
How to depicty Bohr's model
- Circle energy levels
- Normal energy levels
Both should be the same...
What Bohr's model tells us
- Absorption spectra: the missing lines in the absorption spectra correspond to the energies of light that a given atom is capable of absorbing due to the energy differences between its electron's orbits.
- Emission spectra: includes all of the absorption spectral lines because these correspond to the energies emitted as the electron transitions from a higher to lower energy state.
- Unique spectra: as the energy level for atoms are fixed and unique to each atom, the energy level differences are also fixed and unique to each element. Thus, each element has a different and unique emission and absorption spectra as the range of frequencies of light emitted are based on this unique set of energy level differences.
