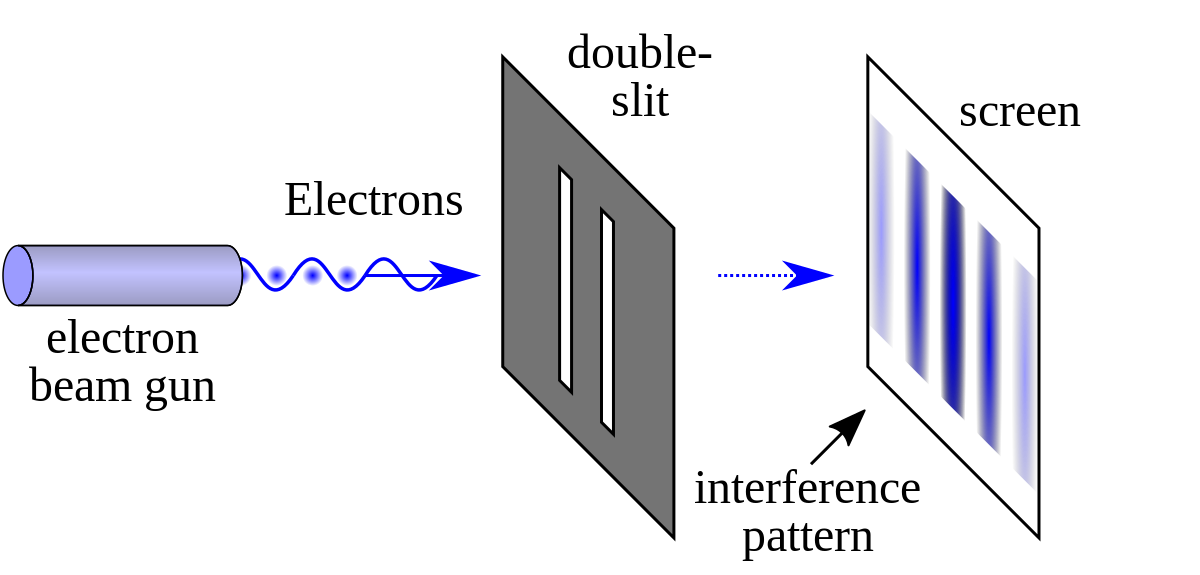
This resulted in many experiments, such as the double slit experiment for electrons.
Gold foil question:
Tada!
Light behaves as waves or particle...
Matter behaves as waves or particles?
e.g. electrons -
de Broglie wavelength:
e.g.
$$\begin{align} \lambda&=\frac{6.63\times 10^{-34}}{9.11 \times 10^{-31}\times 10^6} \\ &=7.27\times 10^ {-10}m \end{align} $$\ Thus, an electron moving at $10^6ms^{-1}$ has a wavelength of $7.27\times 10^{-10}$. Notice this is much smaller than visible light. This small wavelength is apparently useful in the electron microscope, as they can interact with much smaller particles than visible light(better compared to conventional light microscopes). $$