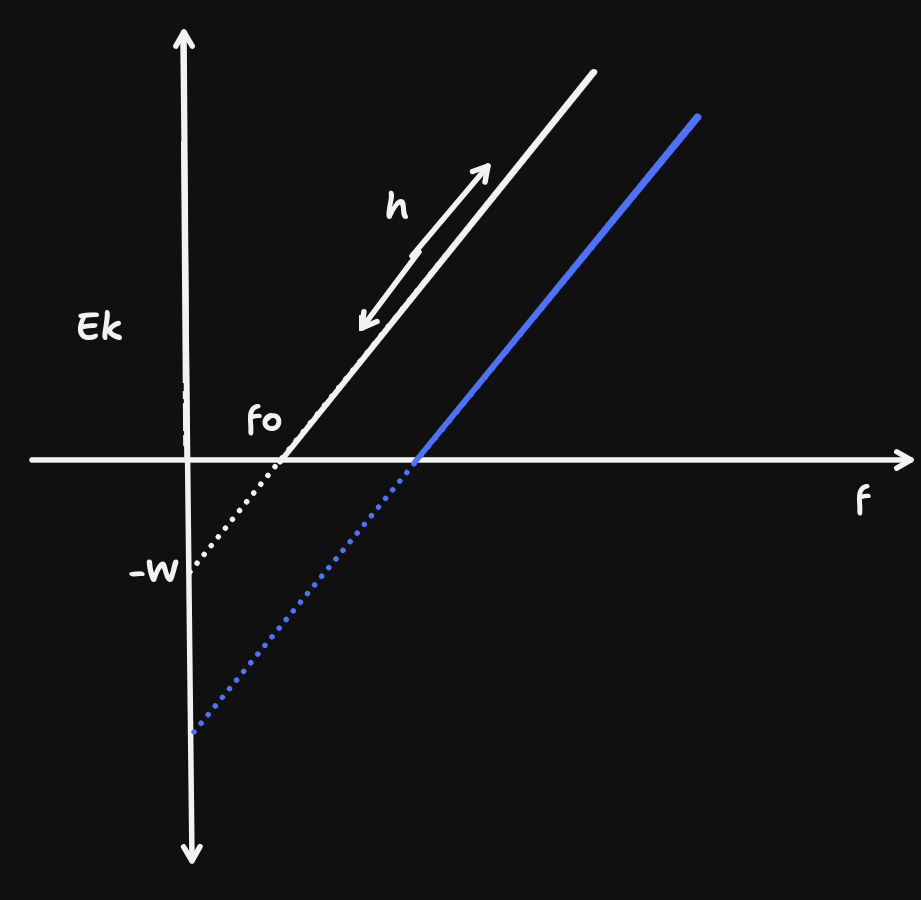
The metal in the metal plate has a unique work function. This because there exists a threshold frequency for the photoelectric effect to occur, which is unique to each metal.
Work function is the minimum energy required to remove the most loosely held electron.
We use the formula $E_{k}=hf-hf_{0}\implies E_{k}=hf-W,$ where $W=hf_{0}$, i.e. the work function. Recall that $f_{0}$ is the threshold frequency.
For example, if $f_{0\text{ sodium}}=5.8\times 10^{14}$, then $W_{\text{sodium}}=5.8\times 10^{14}\times 6.63 \times 10^{-34}=3.85\times 10^{-19}J=2.40eV$
Remember that $c=\lambda f$, hence, $\lambda=\frac{c}{f}=\frac{3\times 10^8}{5.8 \times 10^{14}}=520nm$, hence any photons with wavelength smaller than $520nm$ will produce a photo-electron.
If $UV$ ($240nm$) is shone onto Sodium what is the max $E_{k}$ of the ejected electron? Recall that $W_{\text{sodium}}=3.85\times 10^{-19}J$.
$$\begin{align} E_{k}&=hf-W \\ \therefore E_{k}&=\frac{hc}{\lambda}-W \\ E_{k}&=\frac{6.63\times 10^{-34}\times 3 \times 10^{8}}{240\times 10^{-9}}-3.86\times 10^{-19} \\ &=4.44\times 10^{-19}J \\ \therefore E_{k}&=\frac{1}{2}mv^2 \\ \implies v&=\sqrt{ \frac{2E_{k}}{m} } \\ &=\sqrt{ \frac{2\times 4.44\times 10^{-19}}{9.11 \times 10^-31} } \\ &=9.88 \times 10^{5}ms^{-1} \end{align} $$