Left-click: rotate, Mouse-wheel/middle-click: zoom, Right-click: pan, Escape: close
Coulomb's Law
$$F=\frac{kq_{1}q_{2}}{r^{2}}$$
Where $k$ is a constant, specifically $k=\frac{1}{4\pi\epsilon_{o}}$.
- $k\approx8.99\times10^{9}$
- $\epsilon_{0}$ is a fundamental physical constant, called the permittivity of free space. I.e. how prone to supporting an electric field a material is.
- $\epsilon_{0}=8.85-10^{-12} Fm^{-1}$
The distance between the charges is $r$, of units $m$.
Example 1
- Object 1, $+1C$.
- Object 2, $+2C$.
- Distance is $5m$.
$$\begin{align}
F&=\frac{kq_{1}q_{2}}{r^{2}} \\
&= 8.99\times 10^{9}\times \frac{1\times 2}{5^{2}} \\
&=7.19\times 10^{8} N \text{ away from each other} \\
\end{align}
$$
Note the direction of $F$ is away, as they are equal charges.
Very unrealistic answer(but correct), because this repulsion means its incredibly hard to just have these charges next to each other in such proximity.
Normal static charges is usually $\times 10^{-8}C$.
Note that this force can be paired with $F_{g}$ into cool qs.
Example 2
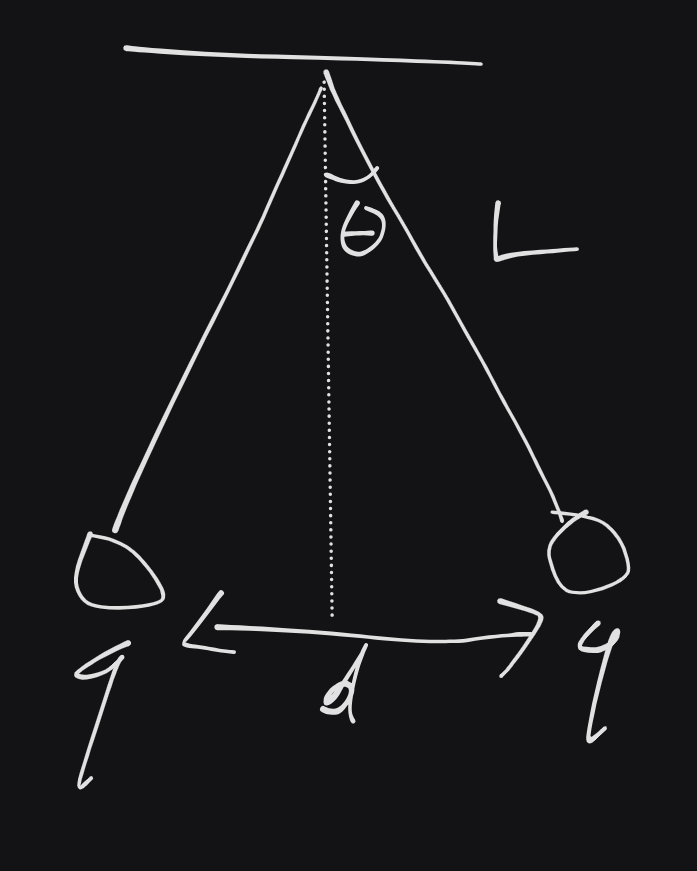
- $L=10cm$
- $d=2cm$
- $m=0.12g$
Find $q$ (equal).
$$\begin{align}
\theta = \sin^{-1}\left( \frac{1}{10} \right)= 5,74°
\end{align}
$$
$$\begin{align}
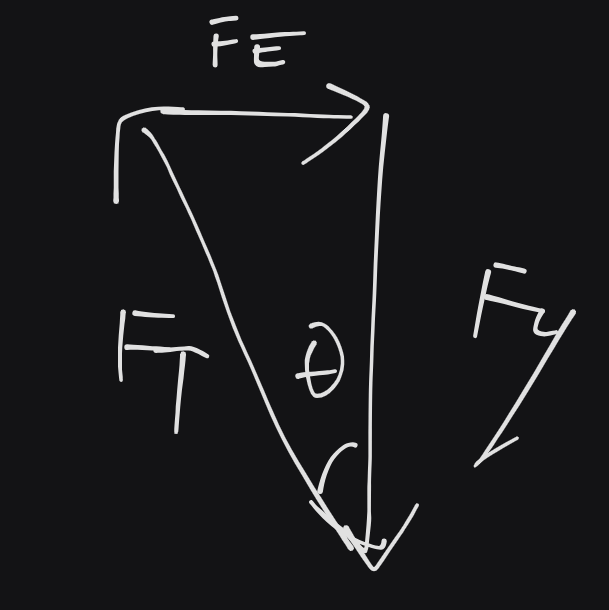
F_{g}=0.12\times{1}0^{-3}\times 9.8 &= 1.18\times 10^{-3}N \\
\therefore \tan\theta&=\frac{F_{E}}{F_{g}} \\
&\implies F_{E}=1.18\times 10^{-4}N \\
\end{align}
$$
$$\begin{align}
F&=\frac{kq_{1}q_{2}}{r^{2}} \\
q_{1}&=q_{2} \\
\therefore q_{1}&=\frac{Fr^{2}}{k} \\
\therefore q_{1}, q_{2}&=\pm{2}.29\times 10^{-8}C
\end{align}
$$

