Left-click: rotate, Mouse-wheel/middle-click: zoom, Right-click: pan, Escape: close
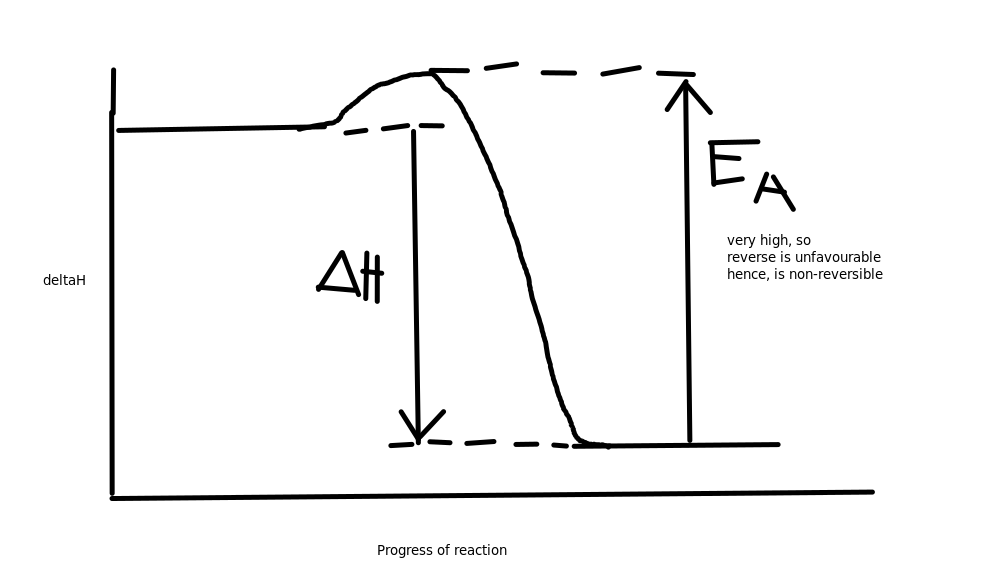
Reversible vs Non-reversible reactions
Most reactions have an energy profile diagram such that the activation energy for both the forward and reverse reaction are reasonably low, and close to each other.
The reactions that don't are considered non-reversible, such that the forward(could be reverse, depends how you look at it) reaction is so much more favourable than the reverse so it occurs to completion. This is because the reverse reaction has too high of an activation energy to occur.
Equilibrium graphs
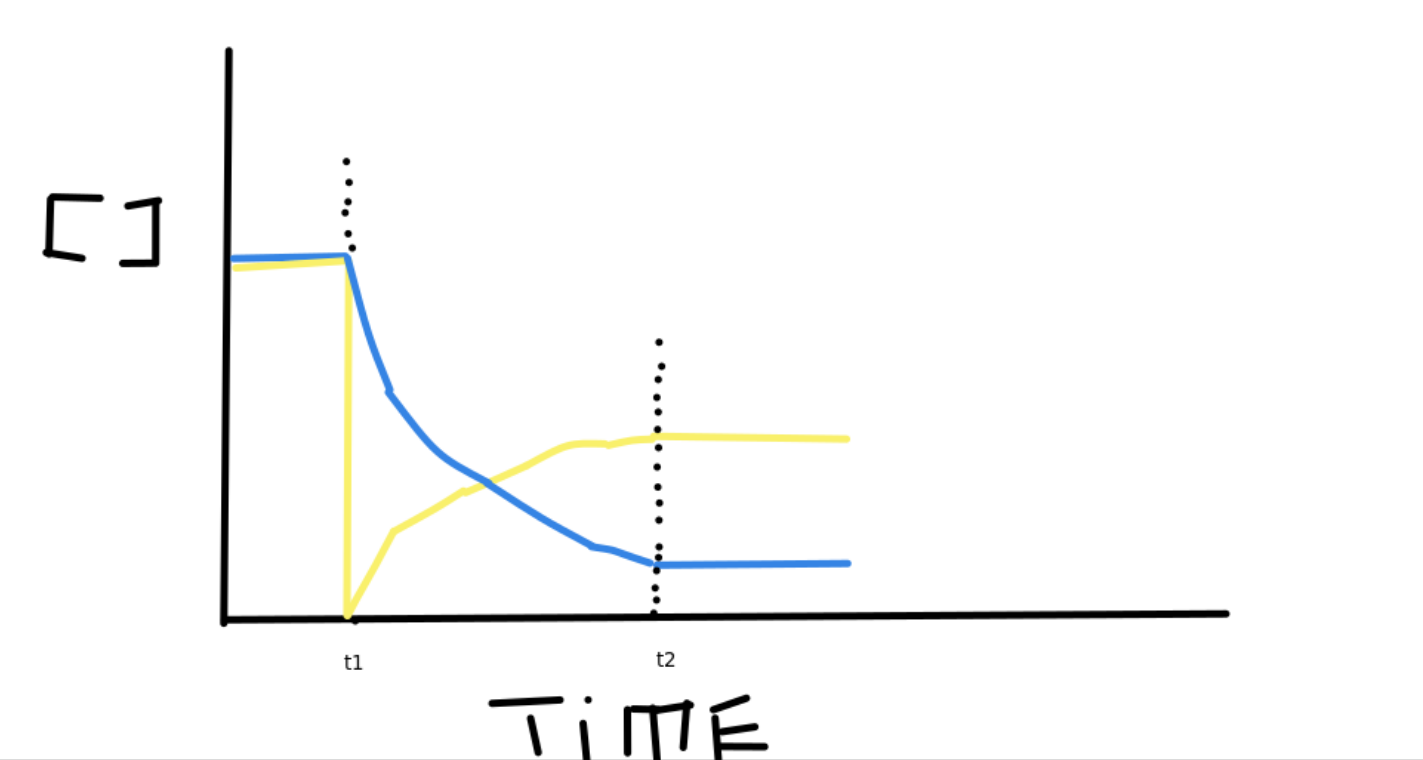
Equilibrium Constant
- $K_{c} = \frac{[C]^c[D]^d}{[A]^a[B]^b}$
- for system $aA + bB \rightleftharpoons cC + dD$
- $K_{c}$ only changes by temperature.
- $K_{c}$ is only used to calculate using reactants, products that have a concentration.
- Hence, reactants/products such as water, solids, liquids do not have a discrete concentration, hence they are not included.
- It follows that only gasses and aqueous substances have concentration, hence only these things can be used in the $K_{c}$.
Example:
$N_{2(g)} + 3H_{2(g)} \rightleftharpoons 2NH_{3(g)}$
Hence.
$K_{c} = \frac{[NH_{3}]^2}{[N_{2}][H_{2}]^3}$
- Marking key says you must write "$K_{c}=$" to get full credit.
$Ca_{(aq)}^{2+} + CO_{3(aq)}^{2-} \rightleftharpoons CaCO_{3(s)}$
Hence,
$K_{c} = \frac{1}{[Ca^{2+}][CO_{3}^{2-}]}$
Why is Kc useful?
- High $K_{c}$
- products favoured
- reaction close to completion
- Low $K_{c}$
- Reactants favoured
- Reaction barely progresses
Two types of reaction
- Heterogeneous
- Different states
- Only include gasses and solutions
- Liquids and solids are written as 1
- Homogeneous
-
e.g. only gasses, only liquid, etc.
-
All reactants and products are included, even if it is all not gasses/aqueous
-
e.g. $A_{(l)} + B_{(l)} \rightleftharpoons C_{(l)}$
- $K_{c} = \frac{[C]}{[A][B]}$
-
[] denotes relative proportions in the liquid mix rather than concentration.
Kw: The special Kc
- $K_{w} = [H^+][OH^{-}]=1\times10^{-14}$ at $25\degree C$
- Because the system is: $H_{2}O \rightleftharpoons H^+{(aq)}+OH^-{{(aq)}}$
- Note that since water is a liquid, it is equal to zero, hence the Kw follows.
Qc
- $K_{c}$ for a system not at equilibrium.
- Literally the same formula.
- If we have a system that is still changing, we can use the data given to create the Qc, which tells us how close the system is to equilibrium.
- When a system is at equilibrium. $Q_{c} = K_{c}$
Example:
-
Assume the $K_{c}$ for the Haber process = 0.6 at $40\degree C$
-
If we had a system approaching equilibrium and we put it into the $K_{C}$ calc, we get $Q_{c}$.
-
Say that $Q_{C}$ = 0.1
-
This implies that the system is not in equilibrium.
-
Since the $Q_{c}$ is lower than $K_{c}$
- There are more reagents then there should be for the system to be in equilibrium
- The forward rate of reaction is currently favoured
- Hence the reagents will decrease and products will increase
- This results in the concentrations of the reagents to fall, and the concentrations of the products to increase, and according to the $K_{c}$ formula, the $Q_{c}$ should rise to become equal to $K_{c}$.
-
If $Q_{c}$ is greater than $K_{c}$,
- There are more products then there should be for the system to be in equilibrium
- The reverse reaction rate is currently favoured
- Hence the reagents will increase and the products will decrease
- This results in the concentration of the reagents to rise, and the concentrations of the products to fall, hence according to the $K_{c}$ formula, the $Q_{c}$ should fall to become equal to $K_{c}$
Rough explanation
- When $Q_{c}$ > $K_{c}$,
-
The products must thus have a greater concentration than at equilibrium, and the reagents must thus have a lower concentration than at equilibrium
-
We know greater/lower concentrations result in greater/lower reaction rates
-
Hence we can infer the immediate forward reaction rate is lower than the immediate reverse reaction rate
-
Hence more products are consumed then produced, lowering the concentration of products partially
-
Similar occurs for the reagents
-
This occurs until the rates of reaction are equal
- The rates of reaction will change as the concentrations change. Since the products are being consumed, the reverse rate of reaction falls, and likewise for the forward rate of reaction
-
At this point, they are in equilibrium.

