long molecules - form plastics, synthetic fibres etc. (1000's of C's)
Simple monomers with double bonds are combined, attaching to each other by breaking the double bond. By breaking the double bond, we bond monomers to each end of a monomers, resulting in the repeating long chain.
e.g. ethene $\implies$ polyethylene
chloroethene $\implies$ polyvinyl chloride, or PVC.
$Cl$ has more electrons. Dispersion between molecules larger, $\therefore$ different properties.
Order doesn't matter cause monomers can be added in many orientations. e.g. for PVC, it could go (top of monomer) H, Cl, Cl, H, not H, Cl, H, Cl as one would assume.
Propene (monomers) $\implies$ polypropylene (same as polypropene)
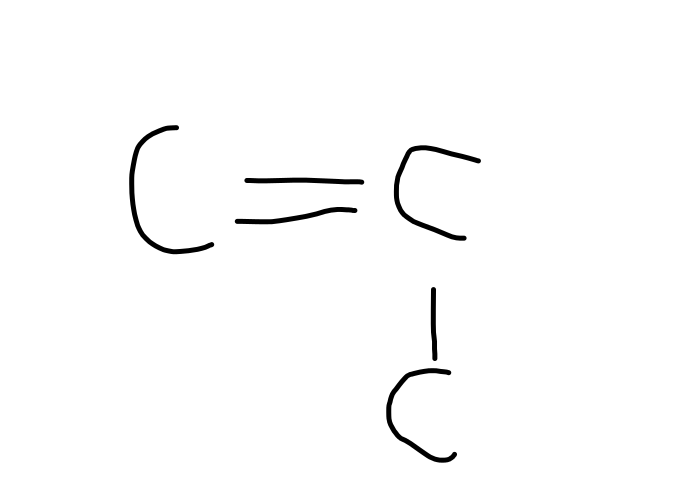
tetrafluoroethene $\implies$ TEFLON!!!!
Intermolecular forces between polymer molecules determine their properties.
Density
Cross linking