Left-click: rotate, Mouse-wheel/middle-click: zoom, Right-click: pan, Escape: close
Production of esters:
- Carboxylic acid + alcohol $\rightleftharpoons$ ester
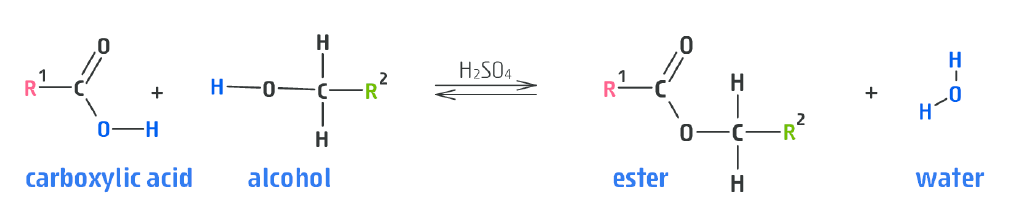
Notice how the resultant ester has the alcohol first, then the carboxylic acid, for the carbon lengths.
- I.e. suppose we reacted ethanoic acid with methanol. The product would be methyl ethanoate.
- You can think about this at the carboxylic acid becoming an "oate" ion, i.e. it loses the $H$ from the $OH$ section of the $COOH$ group.
This kind of reaction is called condensation
Properties
- Polar, due to the double bonded oxygen, and reasonably polar C-O single bond.
- Cannot form hydrogen bonds with itself, but can form hydrogen bonds with water, i.e. molecules with hydrogen (bonded to NOF).
- As it cannot form hydrogen bonds with itself, it has a low M.P/B.P. But it can form H bonds with water, hence it is reasonably soluble.
- Less soluble than alcohols, carboxylic acids, since their hydrogen bonding capacity is decreased, but since they can form some/half of a hydrogen bond they are more soluble than for example propane.
Reversible reaction
- Warm the ester with $H_{2}SO_{4}$ the reverse reaction will occur.
- This is called hydrolysis!
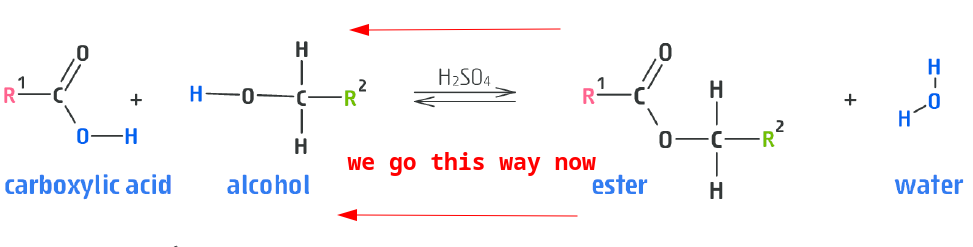
- Warm with $OH^{-}$ (e.g. $NaOH$) gives alcohol back and carboxylic acid salt ion!
- i.e. we have neutralised the acid, we have the resultant "oate" ion.
- e.g. $CH_{3}COOH$ + $OH^{-}$ $\to$ $CH_{3}OH$ + $CH_{3}COO^{-}$
Observations
- Two layers form (generally)
- Due to limited solubility of ester in water (though as before more soluble than your usual non-polar stuff)
- Sweet smelling odour.

