Left-click: rotate, Mouse-wheel/middle-click: zoom, Right-click: pan, Escape: close
Corrosion
Metals can react with $O_{2}$
e.g. $4Al + 3O_{2} \to 2Al_{2}O_{3}$
The formation of $Al_{2}O_{3}$ means that pure $Al$ metal is sealed off from the oxygen and prevents further oxidation.
It forms an oxide layer, which protects the metal from further oxidation.
$Fe$ with $O_{2}$ and $H_{2}O$
- $Fe \to Fe^{2+} + 2\overline{e}$ ox
- $O_{2} + 2H_{2}O + 4\overline{e} \to 4OH^{-}$ red
- Using some sort of basic indicator (phenolphthalein) can be used to identify corrosion, as it produces hydroxide.
Then:
- $Fe^{2+}+ 2OH^{-} \to Fe(OH)_{2(s)}$
- Notice $Fe^{2+}$ is green.
- $Fe(OH){2} + OH^{-} \to Fe(OH){3} + \overline{e}$
- $Fe(OH){3} \to Fe{2}O_{3} \ x \ H_{2}O$
- This is a red, flaky rust
- Flaky is important: the strength of the metal has severely degraded. If the metal was used within structures, the structural integrity decreases significantly.
- This is a problem, as iron is used everywhere
Prevention
Seal - Paint, grease, etc
- Not that great. Any crack or hole in the seal will allow rusting to occur.
Alloy - e.g. Stainless steel
- Better oxidisers within the metal structure, hence rusting does not occur.
- Expensive, hence not a good long term situation.
Coat with $Zn$ - Galvanisation
- This is good as you both seal the substance, and even if it's scratched, the $Zn$ oxidises preferentially.
- i.e. Seals and offers a sacrificial anode.
Coat with $Sn$
- Tin good cause it doesn't react with your food.
- Good seal, but not good as something to oxidise preferentially. If the coating is breached, the iron oxidises, furthermore the $Sn$ extends the electrical conductivity of the metal, accelerating the oxidation of iron.
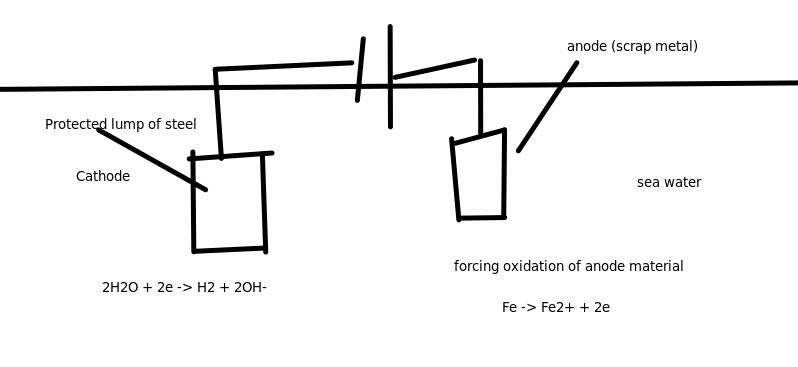
Sacrificial Anode: $Mg$ or $Zn$
- Often found in boats/hot water system.
Cathodic Protection