Left-click: rotate, Mouse-wheel/middle-click: zoom, Right-click: pan, Escape: close
Particles need minimum amount of energy to collide successfully and react, this is called activation energy.
We try to increase rate of reaction because its useful.
- This is done by reducing activation energy, or giving particles enough energy.
Rate of reaction depends on 2 things:
- Frequency of collisions between particles
- Energy with which particles collide
- Orientation of particles (not important)
If particles collide without this energy, they simply bounce off each other. If ideal gas, this is elastic.
Anything that increases the number of successful collisions will speed up a reaction.
How to speed up reaction?
- Increase temperature - this is usually costly, but it will increase rate. This is economically/environmentally unfavourable.
- Velocity of particles increases, therefore particles collide more frequently, and thus more successful reactions occur.
- Add catalyst - this reduces activation energy, and thus more particles will have the required energy to collide successfully, resulting in more success collisions and thus rate of reaction increases
- This is the best solution!! :)
- Increase concentration
- If volume is constant, but more gas is injected, pressure increases. Furthermore, as there are more particles, more particles will collide at a greater frequency. This increases activation energy.
- Increase surface area of solid reactants
High temp give fast rate of reaction but low yield.
- Agitation works, but is not important. i.e. stirring
What is rate of reaction:
- Rate = $\frac{\Delta [concentration]}{\Delta time}$
- Note that rate of reaction creates a decay graph over time.
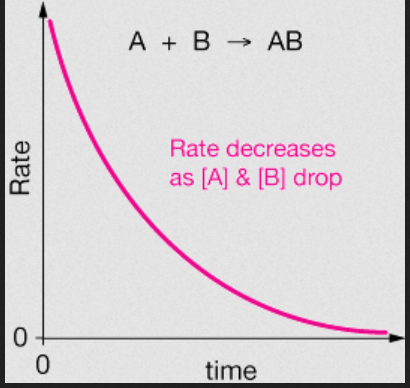
-
Concentration of products is a growth graph that has a horizontal asymptote.
-
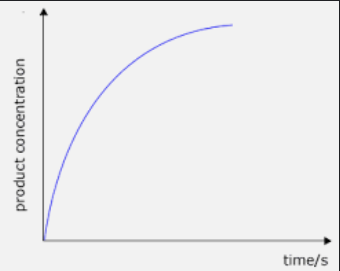
-
No change in concentration of reactants of products, reaction stopped => we ran out of reactants.
-
Concentration increases rapidly initially, rate of reaction is high due to high concentration of reactants (higher frequency of successful collisions)

