Write electronic configuration using s,p,d notation for:
S -> $1s^22s^22p^63s^23p^4$
$S^{-2}$ -> $1s^22s^22p^63s^23p^6$
Ar -> $1s^22s^22p^63s^23p^6$
The last 2 are "Isoelectronic" species/particles - not atoms, because ions are not atoms
What does it mean to be a 'x'-block element?
Their outermost ==subshell== where their valence electrons are found is the 'x' shell.
e.g. Argon is a p-block element, looking above its last subshell is p.
Note: 1s2s2p3s3p4s3d – here, 4s actually has a lower subshell energy than 3d,
As you move along the period, e.g. from Sodium to Argon,
Nuclear charge increases.
Atomic radius decreases?!!!
The principal quantum number (energy shell) stays the same for elements in a period.
Increasing the number of electrons in the inner shells will shield outermost electrons from occupying the outermost principal quantum number .
As nuclear charge increases, electrons in n=1 become closer. This applies for all other shells.
e.g. Na has an atomic charge of 11. In comparison, Argon has a charge of 18. Because of this, it will attract the electrons in n=1 better (electrostatic attraction increases)
Electronegativity: Is the ability for an atom to attract a bonding pair of electrons in a covalent bond.
Covalent bond: shared pair of electrons in a chemical bond
Valence electrons of non-metals that are not shared in the chemical bond are called lone pairs. /non bonding
HCl is a polar molecule.
Hydrogen has no shielding, whereas chlorine has 10 electrons of shielding. Thus, hydrogen's nucleus is more attracted to chlorine's electrons than chlorine is to its. Therefore, chlorine pulls hydrogen towards its nucleus closer than hydrogen pulls chlorine.
We would write chlorine as $\delta -$ , as since it pulls hydrogen closer the electron shared is closer to chlorine, and we write hydrogen as $\delta +$, as its partially lost its electron.
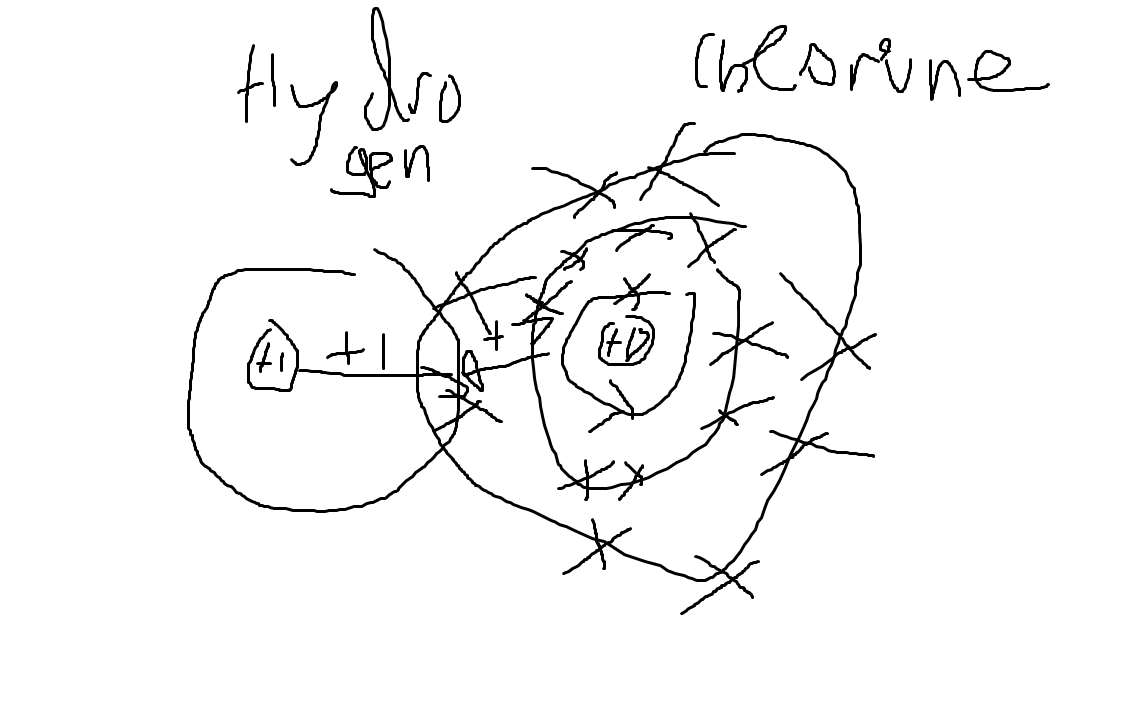
Widjaja liked my diagram a lot. Actually, I think he called me "mentally insane" or something along the lines of those words. Thanks Widjaja ❤️😘
Here, hydrogen has a weaker positive nuclear attractive force than chlorine (10 of its charge is shielded by it), so it does not attract the bonding pair as much as chlorine