STAWA Set 4 Q4
Don’t use moles unless you’re doing calculations
Use:
Mass (g)
Volume (mL)
concentration (mol $L^{-1}$)
Example of mixture to separate:
Sand, NaCl and CuCl_2
Atomic Spectra
Bohr’s model helped describe the spectra of hydrogen.
It showed 4 lines of absorption.
What does spectra represent?
It represents different wavelengths which correlate to different colours.
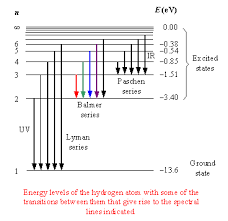
NOTE: Electrons occupy fixed energy levels.
The lines are energy levels.
Lines inside absorption/emissions spectrum are called spectra.
Absorption spectrum: Black lines on coloured spectrum Emissions spectrum: Coloured lines on black spectrum
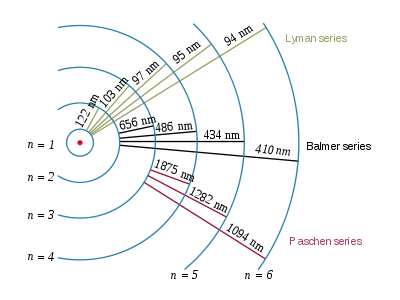
n = 1 → n = 2 does not exist in visible light. (infared)
Balmer series refers to the possible promotions of electrons that produce visible light.
Balmer goes from n = 2 to whatever the largest electron shell is.
Further Reading:
Particle Theory of Light
First proposed by Einstein, he called them photons.
Energy of photon related to frequency of light wave by the Planck constant
In essence, light acts as both a wave and a particle, the wave part being described by Schrodinger's equation when excited electrons return to their ground state.
https://www.youtube.com/watch?v=GhAn8x7Q-d8
Things that excite electrons:
Each atom has a particular pattern for absorption and emissions
Some methods aren’t good, they don’t give enough energy or energy at the exact magnitude/wavelength to excite an electron.
Electrons can absorb energy, and become excited (promoted to a higher energy state) by:
Then they emit energy as electromagnetic radiation, and return to their ground state.
They will always emit the same amount of energy as they absorb.
Balmer series = promotion from n = 2 to other energy levels, which absorb/emit visible light.
Bohr’s Model of the Atom:
Continuous spectrum (white light) vs Emissions spectrum vs Absorption spectrum.
Continuous spectrum is what you see when you use a spectroscope on white light.
How to get continuous spectrum? Iridescent light (source of white light), which does through diffraction grating to create a continuous spectrum
Heating up gas creates an emission spectrum. - heating up gas will give electrons energy,
Lighting white light through cold gas will create an absorption spectrum.
Absorption spectra:
Atomic absorption spectroscopy: - more detail
Emission spectra:
The Bohr Model of the atom and Atomic Emission Spectra: Atomic Structure tutorial | Crash Chemistry
https://www.youtube.com/watch?v=apuWi_Fbtys
Plank discovered that Light energy is quantised.
Einstein discovered that light waves have the properties of a particle.