Properties of salt bridge
- High concentration
- Doesn't form precipitates
- Doesn't react with anything in cell
$E°{\text{cell}} \neq \text{Voltage!}$
In fact, $Voltage = \mid E°{\text{cell}} \mid$
When claiming a salt bridge forms precipitates, write equation.
When writing observations for redox, label observation for anode, observations for cathode.
$$\begin{align}
\text{Cl_{2(aq)}} \text{ half-cell}: \\
\text{Greenish-yellow, pungent effervescent is bubbled into a colourless solution} \\
\text{Loses its effervescence} \implies \text{ colourless solution}
\end{align}
$$
Corrosion - metal oxidises
Wet corrosion - when a liquid is present
Rusting of iron -> when exposed to $H_{2}O,O_{2}$
$$\begin{align}
\text{Oxi: } Fe_{(s)} \to Fe^{2+}_{(aq)} + 2\overline{e } \\
\text{Red: } O_{2(g)} + H_{2}O_{(l)} + 2\overline{e} \to 2OH^{-}_{(aq)} \\
\text{Overall: } Fe_{(s)} + H_{2}O_{(l)} \to Fe(OH)_{2(aq)}
\end{align}
$$
Protection from corrision
- Surface - prevent direct contact
- Plating
- Less reactive metals used to protect $\implies$ lower SOP
- i.e. cover iron with copper coating
- But there is a scratching problem!
- If scratched and exposed, since iron has higher SOP value $\implies$ oxidises preferentially $\implies$ accelerated corrosion
- cathodic
- forcing protected site to become cathode $\implies$ site of reduction by applying a voltage
- thus other site will be anode $\implies$ side of oxidation
- positives
- not cheap
- check teachable again
- sacrificial anode
- attach a metal with a lower SRP to the thing we want to protect electrically
- needs replacing
- but comes at lower cost
- galvanisation
- more reactive metal, higher SOP
- acts as direct contact barrier for protection
- THEN acts as a sacrifical anode
- even if scratching occurs and the thing we want to protect1 is exposed, as long as direct contact is held, electrons can thus flow, so the galvanising material will still oxidise preferentially, i.e. it still is sacrifical anode
see q 109
dynamic equilibrium
- macroscopic properties constant
- temperature, pressure, colour, conc, partial pressure
- forward rate = reverse rate so it appears static
RATE DETERMINING STEP(FOR A REACTION) IS THE ONE WITH THE HIGHEST ACTIVATION ENERGY (I.E. LOWEST REACTION RATE)
when eqi re-established on graph, write $t_{\text{equilibrium re-established}}$2
q: can you reduce the volume of a solution, to increase concentration of all ions?3
to be strong: $K_{a} > 1$
memorise all the funny strong acid strong base, weak acid strong base, etc. etc. graphs for ph curves titrations
talking about amino acid in pH, need to mention:
Cleaning Action Soap question
- Diagram
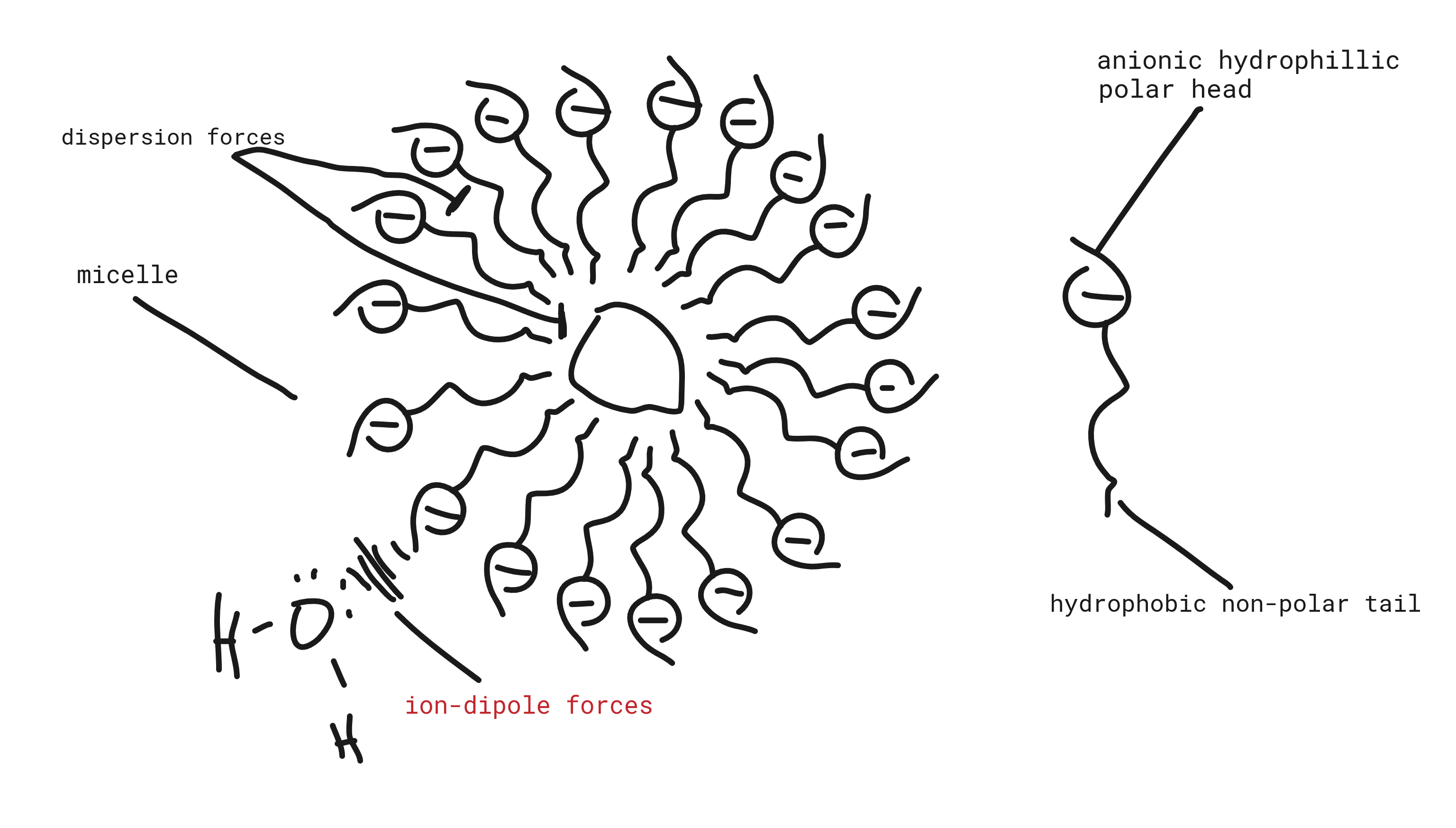
- Soap/detergent has a hydrophillic polar head, i.e. carbonylate/sulfonate ion, and a hydrophobic non-polar tail, i.e. long hydrocarbon chain.
- Tail forms dispersion forces with grease and with other tails, due to their non-polarity
- Head forms ion-dipole forces with water, making it water soluble
- Micelle is formed as soap molecules aggregate around grease, containing it
- Micelle is water soluble and removed with agitation
